Brochosomes (Gr. brochos, the mesh of a net, and soma, the body) are intricately shaped proteinaceous secretory particles of 0.2–20 µm in size produced by the Malpighian tubules of leafhoppers (Cicadellidae), one of the most diverse and abundant insect families.
After molting, leafhoppers release the brochosome-containing secretion through the hindgut and apply it to the fresh integument. The resulting particulate coat apparently prevents leafhoppers from getting trapped in their sticky excrement or water. Some leafhoppers also use brochosomes to cover the egg chambers made by ovipositing females in plant tissues.
Brochosomes are among the most unusual structures produced in living cells. They provide an interesting model system for studies of cell biology, as well as evolutionary studies. Because of their unique structure and properties, brochosomes may have applications in science and technology.
Brochosomes are a unique evolutionary innovation of leafhoppers (Cicadellidae) that has contributed to the spectacular diversification of this family. Studies of brochosomes and related unusual morphological traits and behaviors of leafhoppers provide important insights into the biology, ecology, systematics, and evolution of these insects.
Although brochosomes were discovered more than 50 years ago (Tulloch et al. 1952) , very little is known about the chemical composition of brochosomes, the mechanisms involved in their development, and their function.
Structure and Diversity of Brochosomes
The leafhopper family includes ca. 25,000 described species, and perhaps more await description. The current knowledge of brochosomes is based on only a fraction of this diversity that has been studied thus far under an electron microscope. Brochosomes have been found in nearly all studied species, leaving no doubts that they are produced in the majority of leafhoppers. The size, shape, fine details of the surface, and internal structure of brochosomes all vary dramatically. Brochosomes produced by different species can look identical or very different. Moreover, in certain species, different morphological types of brochosomes are produced simultaneously or at different stages of development.
| Integumental brochosomes | Egg brochosomes | |
| Examples |  |
 |
| Use | Distributed across the integument. | Placed onto egg nests made in plant tissues or directly onto eggs. |
| Distribution | All leafhoppers. Worldwide. | A small group of about 200 related species from the tribe Proconiini. New World. |
| Produced by | Males and females, often also nymphs | Gravid females only |
| Function | Protection from excrement and water? | Protection of eggs? |
| Size | 0.2 to 5.0 µm | 1.0 to 20.0 µm |
| Shape | Spherical | Elongate, rarely spherical |
Composition of Brochosomes
Brochosomes are extraordinarily stable structures. They are insoluble in water and organic solvents, cold or boiling. They are resistant to heat. Dry masses of brochosomes are highly hydrophobic. Previous studies have indicated that brochosomes contain both protein and lipid components. This conclusion has been based on positive staining by certain histochemical dyes observed using the light microsope (Smith & Littau 1960; Gouranton & Maillet 1968) and electron microscopic observations of brochosomes digested with various enzymes (Gouranton & Maillet 1968). Preliminary results of our ongoing study indicate that ca. 60% of the brochosome skeletons comprise glycine-rich proteins. Some known glycine-rich structural proteins of insects account for the strength of silk (fibroin), egg cases, egg chorion, and flexible cuticle (resilin).
Production of Brochosomes
Brochosomes are produced in special modified segments of the Malpighian tubules of leafhoppers. The Malpighian tubules are the main excretory organs of insects, somewhat analagous to kidneys of vertebrates, their primary functions being excretion and ion exchange. However, in some insects the Malpighian tubules or parts of them are modified to produce various materials for external use (cocoons, covers, “spittle,” defense). Production of brochosomes is one of the most remarkable cases of acquiring by these organs a secondary function.
Malpighian tubules of leafhoppers
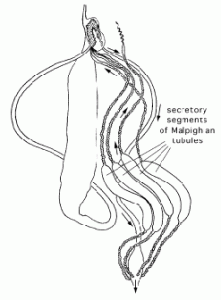 Leafhoppers have four Malpighian tubules. Each tubule has an inflated middle segment comprising secretory cells which produce brochosomes. Other tubule parts serve excretory function. They display the same structure as the regular Malpighian tubules found in most insects. The drawing shows a typical alimentary canal and the Malpighian tubules of a leafhopper. Brochosomes produced in secretory segments of the tubules are released through the hindgut (arrows).
Leafhoppers have four Malpighian tubules. Each tubule has an inflated middle segment comprising secretory cells which produce brochosomes. Other tubule parts serve excretory function. They display the same structure as the regular Malpighian tubules found in most insects. The drawing shows a typical alimentary canal and the Malpighian tubules of a leafhopper. Brochosomes produced in secretory segments of the tubules are released through the hindgut (arrows).
Function of Brochosomes
The function of brochosomes remains poorly understood. Several hypothetical functions have been proposed, but most of them have not yet been tested experimentally (however see Velema et al. 2005 on the role of egg brochosome coats as protection against egg parasitoids).
The Malpighian tubules are primary excretory organs of insects, but in leafhoppers this appears to be the function of only thin parts of the tubules, while the inflated brochosome-manufacturing segments are entirely involved in synthesis. Similar cases of secondary synthetic specializations of the Malpighian tubules are known in various insects. For example, these organs manufacture products such as silks or other components of cocoons (Neuroptera, Rhaphidioptera, some Hymenoptera), defensive secreta (some Neuroptera), components of scale covers (Hemiptera: Diaspididae), substances that agglutinate soil particles (immature Cicadidae) or foam the “spittle” produced by immature spittlebugs (Hemiptera: Cercopoidea).
| Hypothetical functions | Likelihood* |
| Repellence of water and sticky substances (e.g., excrement) | Very likely (esp. integumental brochosomes) |
| Protection against fungal or microbial pathogens | Likely |
| Protection against parasites | Likely (esp. egg brochosomes**) |
| Protection against desiccation | Likely in some cases |
| Thermoregulation, Protection against UV light | Likely in some cases |
| Pheromone reservoirs | Unlikely |
*See Rakitov 2002b for detailed discussion. **See Velema et al. 2005 for experimental evidence.
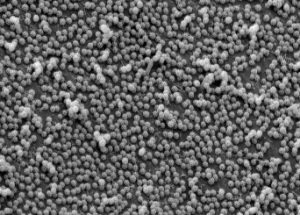
As a rule, body-coating structures (feathers, mammalian hairs, lepidopteran scales, etc. ) have multiple functions. Brochosomes, which form coats on the integument of leafhoppers, have probably evolved multiple functions as well.
Water-repellent properties

Water and water-based liquids do not adhere to brochosome-coated bodies of leafhoppers. The reason for this is a very complex fractal surface geometry formed by the layer of brochosomes. In other animals and plants, the extreme water-repellence is achieved by either development of a surface microsculpture, or secretion of microscopic wax particles. It is an important property for leafhoppers because it protects them from 1) their own copious liquid excretion (filtered plant sap), which is often sticky, 2) rain and dew, and possibly 3) spider webs.
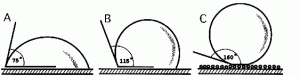
Brochosomes as an alternative to wax
In many other insects, the integument produces tiny chunks of fibrils of wax, forming a water-repellent coat. Although such particulate waxes differ from brochosomes in both chemical nature and origin, the functions of these two biomaterials in many cases appear to be similar. Waxy particulate coats are especially common and diverse among the plant-feeding Hemiptera, other than leafhoppers planthoppers, aphids, whiteflies, and scale insects. Just as leafhoppers, these hemipterans need protection from their sticky sap-derived excretion, as well as from a variety of other hazards. In several such groups, the integumental wax is also used as a coat of the eggs. Unfortunately, as in the case of brochosomes, the properties and the adaptive significance of the particulate waxes have never been studied experimentally and, therefore, remain hypothetical.Read more: Rakitov 2002 b, Velema et al. 2005
Anointing behaviors
The term “anointing” refers to the behaviors displayed by leafhoppers and treehoppers when they release the fluid containing the Malpighian tubule secretory products (brochosomes or other) and apply this fluid onto the body. Anointing is typically observed after molts. The materials applied to the new integument give it additional properties, such as water-repellence. In leafhoppers, dry brochosomes are further distributed across the integument through grooming. Anointing and grooming of leafhoppers are two successive stages of making the brochosome coat. Anointing includes one or several series of stereotyped movements that usually differ between nymphs (immatures) and adults. There are also interspecific differences in anointing behaviors.


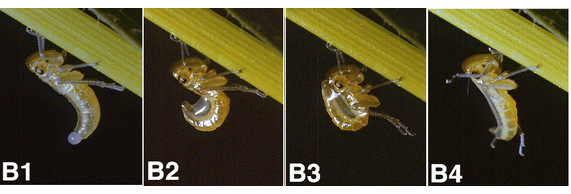

Legs: chaetotaxy
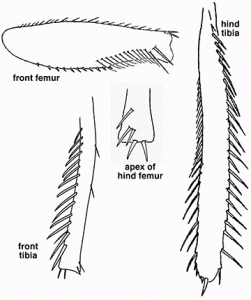 Most leafhoppers use their legs to transfer droplets of the brochosome-containing secretion onto the body (anointing), and then to distribute dry brochosomes over the integument (grooming). A distinctive feature of the leafhopper legs are rows and groups of strong setae (macrosetae), which can be acute, capitate, inflated like balloons, or hooked at the apex. They serve as fine tools for manipulating brochosomes. Read more: Rakitov, 1998.
Most leafhoppers use their legs to transfer droplets of the brochosome-containing secretion onto the body (anointing), and then to distribute dry brochosomes over the integument (grooming). A distinctive feature of the leafhopper legs are rows and groups of strong setae (macrosetae), which can be acute, capitate, inflated like balloons, or hooked at the apex. They serve as fine tools for manipulating brochosomes. Read more: Rakitov, 1998.
Sexual dimorphism
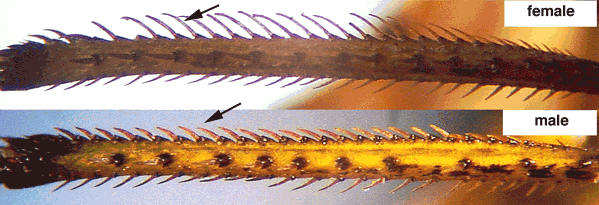 In certain species from the lefhopper tribe Proconiini, females use one of the four longitudinal rows of setae in the hind tibiae to transfer brochosomes from the forewings onto the egg nests. In females of such species, the setae of this row are usually significantly longer than in males.
In certain species from the lefhopper tribe Proconiini, females use one of the four longitudinal rows of setae in the hind tibiae to transfer brochosomes from the forewings onto the egg nests. In females of such species, the setae of this row are usually significantly longer than in males.
Read more: Rakitov 2004.
Brochosomes in Treehoppers
Treehoppers (Membracidae, Aetalionidae, and Melizoderidae) are the nearest known relatives of leafhoppers (Cicadellidae). The integument of treehoppers has no brochosome coat. Several authors have reported finding brochosomes on the integument (Day 1993) or in the Malpighian tubules (Gouranton & Maillet 1967) of certain Membracidae, but in neither case the evidence is substantial. More extensive studies of the treehopper integument (Dietrich 1989) and the Malpighian tubules (Rakitov unpublished) indicated that treehoppers do not produce brochosomes. However, the structure of the Malpighian tubules in Membracidae is almost identical to that in leafhoppers, comprising well developed secretory segments. In both immatures and adults, the cells of these segments synthesize and export products distinct from brochosomes (like in certain leafhopper nymphs, which produce secretory products other than brochosomes). Also like leafhoppers, Membracidae display post-molt anointing behaviors, releasing the Malpighian tubule secretory products and spreading them over the new integument (Rakitov, 1996). These behaviors have been observed so far only in a few species from the tribes Centrotini, Heteronotini, and Platycotini. It remains unknown whether they are characteristic of all membracids. Neither the Malpighian tubules, nor post-molt behaviors have been studied in Aetalionidae and Melizoderidae.
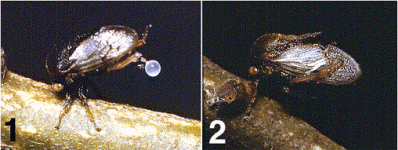

Treehopper nymphs release the Malpighian tubule secretion onto the plant surface and bathe in it . This behavior is identical to the bathing anointing behavior of certain leafhopper nymphs.

Research problems
The function of anointing behaviors in Membracidae remains completely unknown. The Malpighian tubule secretory products of treehoppers do not form any particulate layer on the integument and apparently do not make it water-repellent. Their chemical nature and properties have not been investigated. The leg chaetotaxy in treehoppers is reduced, which correlates with lack of the brochosome coat. According to a recent estimate of the phylogeny, treehoppers must have lost the ability to produce brochosomes secondarily (Dietrich et al. 2001). The ecological conditions which might have favored these changes in the treehopper lineage remain obscure. More extensive studies of the behaviors and the Malpighian tubule secretions in all treehopper families (Membracidae, Aetalionidae, and Melizoderidae) are needed to clarify the biological function and evolution of the Malpighian tubule secretions in this lineage.
Origin and evolution of brochosomes and related behaviors
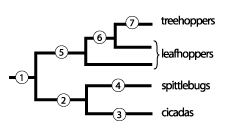
Apparently, both the secretory specializations of the Malpighian tubules and the behaviors in which the tubule products were applied to the integument have already been present in the common ancestor of modern Hemiptera Cicadomorpha: leafhoppers + treehoppers (Membracoidea), spittlebugs (Cercopoidea), and cicadas (Cicadoidea). In the last two groups, these specializations are only present in the immatures, playing important roles in their peculiar life styles.
1 : Ancestor of all Cicadomorpha. Distal parts of the Malpighian tubules were modified for synthesis of proteins, apparently only in the immatures (subterraneous?). The function of these hypothetical products is unknown.
2 : Ancestor of cicadas & spittlebugs. Immatures lived concealed. The distal parts of the immature Malpighian tubules manufactured proteins, and proximal parts mucopolysaccharids. The secretion was mixed into excrement. The specialization disappeared at the adult stage.
3: Cicadas. Immatures live under the ground. The Malpighian tubule secreta are used to strengthen the walls of burrows and possibly also to clean the integument. The anti-fungal function is possible. The specialization disappears at the adult stage.
4: Spittlebugs. Immatures live within their foamed excrement or excrement-filled calcareous dwelling tubes. The Malpighian tubule secreta are used as surfactants stabilizing the foam or as a structural component of the dwelling tubes. The specialization disappears at the adult stage.
5: Leafhoppers. The Malpighian tubules are active as glands in both the immatures and adults. The main secretory product, brochosomes, are released separately from excrement and applied to the integument in anointing and grooming behaviors. Legs are armed with rows and groups of strong setae manipulating the particles. Hypothetical function: water- and excrement-repellence, various other kinds of protection.
6: Leafhoppers (part). The Malpighian tubules of immatures produce non-brochosomal alternative product,s switching to production of brochosomes during the last nymphal instar. Immatures display a peculiar anointing behavior, “bathing.”
7: Treehoppers. Brochosomes lost. The Malpighian tubules produce non-brochosomal alternative secreta in both the immatures and adults. Anointing retained. Immatures display “bathing.”

Research questions
Are brochosomes homologous to the proteinaceous secreta of the cicada and spittlebug immatures?
Unfortunately, the chemical nature of the tubule secretory products is not yet known in enough detail to prove that the same ancestral secretory mechanisms are used in both cases.
Did brochosomes evolve from products with a less complex structure? Did their peculiar geometric structure evolve gradually?
Because all the extant leafhoppers have basically similar brochosomes, nothing is known about the pre-brochosomic period of the leafhopper evolution.
Current Brochosome Projects
Composition and Properties of Brochosomes
The poor knowledge of the composition of brochosomes is a stumbling block in understanding the development, function, and evolution of this unique biomaterial. The project involves development of techniques for the isolation of brochosomes, and the biochemical characterization of this material. The methods include microfiltration, centrifugation, scanning and transmission electron microscopy, electrophoresis, and other methods of protein chemistry.
Homoplastic evolution of a stereotyped behavior and associated structural and physiological traits in the leafhopper genus Cuerna (Insecta: Hemiptera, Cicadellidae)
Rakitov R. A., Dietrich C. H.
Loss of ancestral traits and trait complexes is a pervasive aspect of the evolutionary process, but its causes, mechanisms, consequences, and the possibility of restoration of the lost traits are still poorly understood. The current project aims to explore the patterns of repeated decay and loss of a stereotyped maternal behavior in the North American leafhopper genus Cuerna as a model of the regressive evolution of a complex biological function at the species level. In some of the 31 known species of the genus, females display an ancestral egg-powdering behavior, coating the egg nests with a layer of specialized Malpighian tubule products and displaying a suite of corresponding physiological and structural modifications, while in other species no such behavior is observed. Preliminary phylogenetic analyses suggested that egg-powdering has been lost or lost-and-restored within the genus multiple times. In the three years of the project the investigators will collect detailed comparative data on the egg-laying behavior and associated specializations of the external morphology and the Malpighian tubules for every species of Cuerna. Then, they will analyze these in the context of a detailed DNA-based phylogeny of the genus to address the following questions: Did the changes of particular traits proceed as quantum leaps or gradually? Do morphological, behavioral, and physiological traits display similar evolutionary plasticity? Did they change in a certain order? Did any restorations of lost egg-powdering occur in the evolution of Cuerna? What were the most likely causes of the losses? The study will also result in a taxonomic revision of the genus, which currently contains a number of poorly defined species. The study will contribute to management of economically important species of Cuerna by providing identification aids, as well as behavioral and physiological data for improved integrated pest management. An interactive key to species of Cuerna, complete with bionomical and distribution data, will be served on-line. The project will also provide training for a graduate student in the systematics, molecular phylogenetics, and evolutionary biology of insects.


kaloostiani, gladiola, sayi
Brochosome Bibliography
- Arzone, A. (1986) Brocosomi: origine, forma, funzione. Atti Accad. Naz. Ital. Entomol. Rc. 34: 59-71.
- Azevedo-Filho W. S. & Carvalho G. S. (2005) Brochosomes-for-eggs of the Proconiini (Hemiptera: Cicadellidae, Cicadellinae) species associated with orchards of Citrus sinensis (L.) Osbeck in Rio Grande do Sul, Brazil. Neotropical Entomology 34 (3): 387-394.
- Bigg, E. K. (2003) Brochosomes – a tracer for near-surface air. Atmospheric Research 66: 141-144.
- Cheung, W. W. K. & Purcell A. H. (1991) Brochosome production in the Malpighian tubules of the leafhopper Euscelidius variegatus (Homoptera: Cicadellidae): an electron microscopic study. InProceedings of the 49th Annual Meeting of the Electron Microscopy Society of America, ed. G. W. Bailey, pp. 264-265. San Francisco Press, San Francisco, California.
- Day M. F. (1993) Brochosomes of Australian Cicadelloidea. In: Drosopoulos S., Petrakis P. V., Claridge M. F. and de Vrijer P. W. F. (eds.) Proc. 8th Auchenorrhyncha Congr., Delphi, Greece, 913 Aug. 1993:10-11.
- Day M. F.& A. McKinnon (1951) A study of some aspects of the feeding of the jassid Orosius. Austr. J. Scient. Res. (B). 4: 125?.
- Day M. F. & M. Briggs (1958) The origin and structure of brochosomes. J. Ultrastruct. Res. 2: 239-244.
- Dietrich C. H. (1989) Surface sculpturing of the abdominal integument of Membracidae and other Auchenorrhyncha (Homoptera). Proc. Entomol. Soc. Washington 91: 143-152.
- Dietrich C. H., Rakitov R. A., Holmes J. L. and W. C. Black, IV (2001) Phylogeny of the major lineages of Membracoidea (Insecta: Hemiptera: Cicadomorpha) based on 28S rDNA sequences. Mol. Phyl. Evol.18: 293-305.
- Gouranton J. & Maillet, P. L. (1966) Sur la production de corpuscules lipoprotéiques par les tubes de Malpighi de certains Insectes. C. R. Soc. Biol. 160: 1724-1726.
- Gouranton J. (1967) Presence d’une phosphomonoesterase alcaline liee aux brochosomes dans les tubes de Malpighi de la cicadelle verte. Compt. Rend. Seanc. Soc. Biol. 161: 907-909.
- Gouranton J. & Maillet, P. L. (1967) Origine et structure des brochosomes. J. Microscopie 6: 53-64.
- Gouranton J. (1968) Observations histochimiques et histoenzymologiques sur le tube digestif de quelques Homopteres Cercopides et Jassides. J. Ins. Physiol. 14: 569-579.
- Günthart H. (1977) Einfluss der Insektenalters auf Bestimmungsmerkmale. Biotaxonomische und rasterelektronenmikroskopische Untersuchungen bei Kleinzikaden (Homoptera, Auchenorrhyncha, Cicadellidae). Mitt. Schweiz. Entomol. Ges. 50: 189-201.
- Hix R. L. (2001) Egg-laying and brochosome production observed in glassy-winged sharpshooter.California Agriculture. 50 (4): 19-22.
- Humphrey E. C. & I. Dworakowska (2002) The natural history of brochosomes in Yakuza gaunga(Hemiptera, Auchenorrhyncha, Cicadellidae, Typhlocybinae, Erythroneurini). Denisia 4: 433-454.
- Mayse M. A. (1981) Observations on the occurrence of chalky deposits on forewings of Oncometopia orbona (F.) (Homoptera: Cicadellidae). Proc. Arkansas Acad. Sci. 35: 84-86.
- Navone P. (1987) Origine, struttura e funzioni di escreti e secreti entomatici di aspetto ceroso distribuiti sul corpo mediante zampe. Ann. Fac. Sci. Agr. Univ. Torino 14: 237-294.
- Neville A. C. & Smith D. C. (1970) “Airborne organism” identified. Nature, 225: 199.
- Pollard H. N.& Yonce C. E. (1965) Significance of length of tibial spines relative to oviposition processes by some leafhoppers (Hemiptera: Cicadellidae). Ann. Ent. Soc. Am. 58: 594-595.
- Rakitov R. A. (1992) The leafhopper Vilbasteana oculata (Lindb.) coats its cuticle with a secretion of the Malpighian tubules. Zoologicheski Zhurnal 71: 49-57 (In Russian). [English translation: Entomol. Rev., 1993, 71: 148-157].
- Rakitov R. A. (1995) The covering formed by brochosomes on the cuticle of leafhoppers (Homoptera, Cicadellidae). Zoologicheski Zhurnal 74: 19-32 (in Russian). [English translation in: Entomol. Rev. 1995,74: 90-103].
- Rakitov R. A. (1996) Post-moulting behaviour associated with Malpighian tubule secretions in leafhoppers and treehoppers (Auchenorrhyncha: Membracoidea). Eur. J. Entomol. 93 : 167-184.
- Rakitov R. A. (1998) On differentiation of cicadellid leg chaetotaxy. Russian Ent. J. 6 : 7-27.
- Rakitov R. A. (1999) Secretory products of the Malpighian tubules of Cicadellidae (Hemiptera, Membracoidea): an ultrastructural study. Int. J. Insect Morph. Embryol. 28: 179-192.
- Rakitov R. A. (2000a) Secretion of brochosomes during the ontogenesis of a leafhopper, Oncometopia orbona (F.) (Insecta, Homoptera, Cicadellidae). Tissue & Cell. 32: 28-39.
- Rakitov R. A. (2000b) Nymphal biology and anointing behaviors of Xestocephalus desertorum (Berg), a leafhopper feeding on grass roots. J. N. Y. Entomol. Soc. 108: 171-180.
- Rakitov R. A. (2002a) Structure and function of the Malpighian tubules, and related behaviors of juvenile cicadas: evidence of homology with spittlebugs (Hemiptera, Cicadoidea & Cercopoidea). Zoologischer Anzeiger 241: 117-130.
- Rakitov R. A. (2002b) What are brochosomes for? An enigma of leafhoppers (Hemiptera, Cicadellidae).Denisia 4: 411-432.
- Rakitov R.A. (2004) Powdering of egg nests with brochosomes and related sexual dimorphism in leafhoppers (Insecta, Hemiptera, Cicadellidae). Zoological Journal of the Linnean Society 140: 353-381.
- Rakitov R.A. & Godoy C. (2005) New egg-powdering sharpshooters from Costa Rica. Annals of the Entomological Society of America 98: 444-457.
- Rakitov R.A. (2009) Brochosomal coatings of the integument of leafhoppers (Hemiptera, Cicadellidae). In: S.N. Gorb (ed.), Functional Surfaces in Biology, Vol. 1, 113-137.
- Smith, D. S. & Littau V. G. (1960) Cellular specialization in the excretory epithelia of an insect,Macrosteles fascifrons Stål (Homoptera). J. Cell. Biol. 8: 103-133.
- Storey, H. H. & Nichols R. F. W. (1937) Defaecation by a jassid species. Proc. R. Entomol. Soc. London (A) 12: 149-150.
- Swain, R. B. (1936) Notes on the oviposition and life-history of the leafhopper Oncometopia undataFabr. (Homoptera: Cicadellidae). Ent. News 47: 264-266.
- Tulloch G. S., Shapiro J. E. & Cochran G. W. (1952) The occurrence of ultramicroscopic bodies with leafhoppers and mosquitoes. Bull. Brooklyn Entomol. Soc. 47: 41-42.
- Tulloch G. S. & Shapiro J. E. (1953) Brochosomes. Bull. Brookl. Ent. Soc. 48: 57-63.
- Tulloch G. S. & Shapiro J. E. (1954) Brochosomes and leafhoppers. Science. 120: 232.
- Velema H.-P., Hemerik L., Hoddle M. S. & Luck R. F. (2005) Brochosome influence on parasitisation efficiency of Homalodisca coagulata (Say) (Hemiptera: Cicadellidae) egg masses by Gonatocerus ashmeadi Girault (Hymenoptera: Mymaridae). Ecological Entomology 30: 485-496.
- Vidano, C. & Arzone, A. (1984) “Wax-area” in cicadellids and its connection with brochosomes from Malpighian tubules. Mitt. Schweiz. Entomol. Ges. 57: 444-445.
- Wiffen R. D. & Heard M. J. (1969) Unidentified airborn species. Nature 224: 715.
- Wilde H. A. & Cochran G. W. (1956) Brochosomes on certain species of insects of Western North America. Proc. ent. Soc. Br. Columbia 53: 19-20.
- Wittmaack, K. (2005) Brochosomes produced by leafhoppers – a widely unknown, yet highly abundant species of bioaerosols in ambient air. Atmospheric Environment 39(6): 1173-1180.
- Wyniger D., Burckhardt D., Mühlenthaler R. & Mathys D. (2008) Documentation of brochosomes within Hemiptera, with emphasis on Heteroptera (Insecta). Zoologischer Anzeiger 247: 329-341.

